How does
early life stress change brain development?
How do these changes mediate increased risk for depression
and other psychiatric syndromes?
The broad goal of the lab is to understand mechanistically how early life experiences are encoded and maintained into adulthood to have long-lasting impact on behavior. The lab is currently focused on understanding how early life stress increases sensitivity to subsequent stress. This research is translationally motivated by the robust clinical finding that child maltreatment and other forms of early life stress increase the lifetime risk of depression and other mood, anxiety, and drug disorders. Studies in humans and animals suggest that early life stress sensitizes individuals to stress later in life, leading to a first appearance or synergistic worsening of depression-like symptoms after additional stress.
Mouse models of stress and depression
To study the molecular correlates of lifelong stress vulnerability, we use a “two-hit” stress paradigm in mice in which early life stress in a sensitive window increases susceptibility for depression-like behavior, but only after experience of additional stress in adulthood (Science, 2017). In lieu of a "HAM-D for Mice," we test responses across a battery of tests of depression-like and anxiety-like behavior, taking into account both individual and composite measures.
A major focus of our work is trying to understand the neurobiological mechanisms underlying this latent behavioral vulnerability. We hypothesize that key brain regions are “primed” to respond to additional stress at both cellular and transcriptional/epigenetic levels.
Genome-wide approaches
Our previous work used bulk tissue RNA-sequencing to identify broad and long-lasting transcriptional changes in brain reward regions implicated in depression-like behavior. Two interesting patterns emerged, which we are now investigating. First, even prior to behavioral changes, early life stress programs depression-like gene expression patterns similar to those from mice exhibiting depression-like behavior after adult stress. Second, early life stress programs a latent, unique transcriptional response to adult stress among a subset of genes. These important transcriptional patterns could only be revealed through the use of RNA-seq, highlighting the unique power of such approaches.
We are now working to understand how these patterns emerge and propagate across development in a cell-type-specific manner.
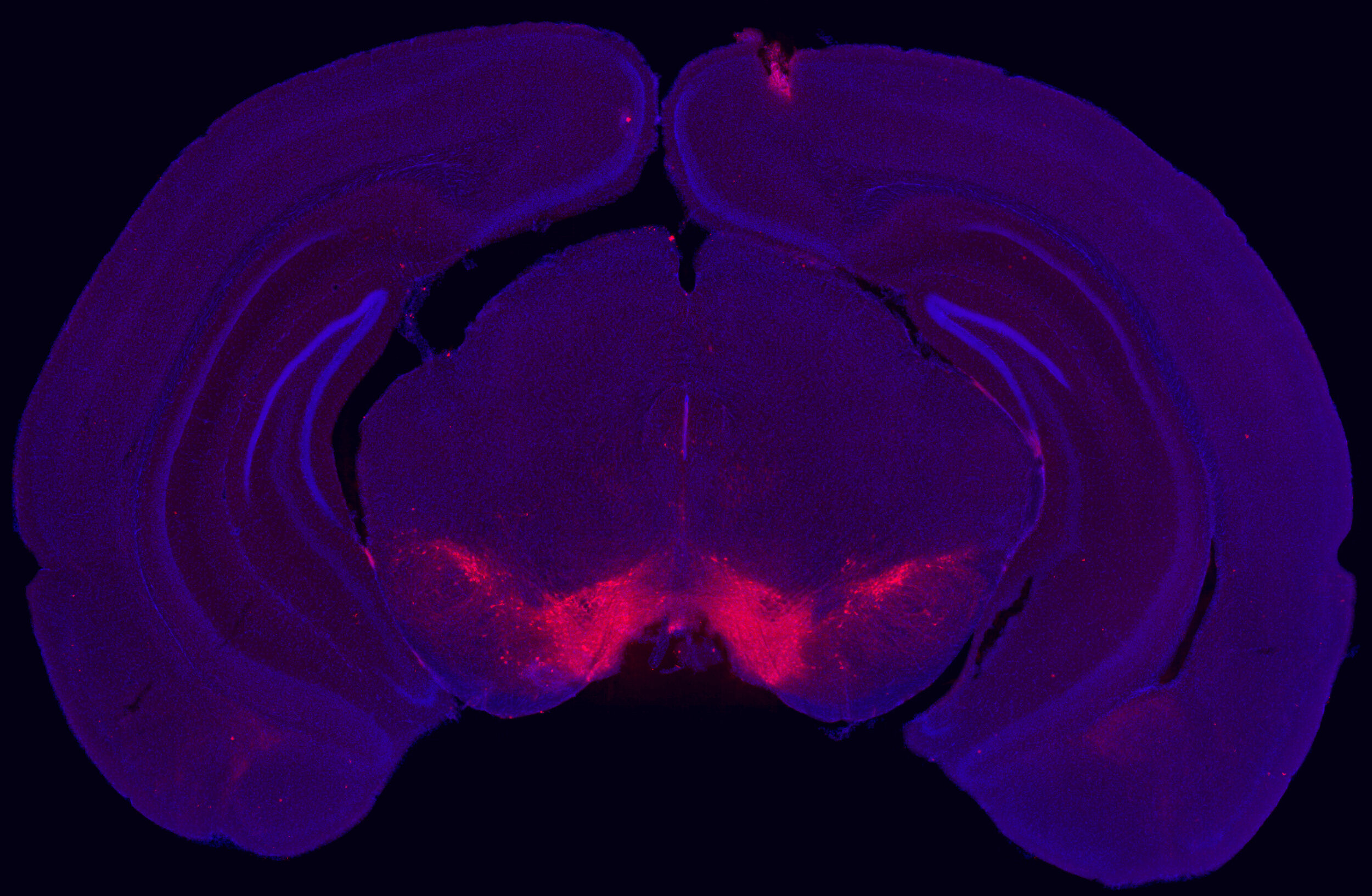
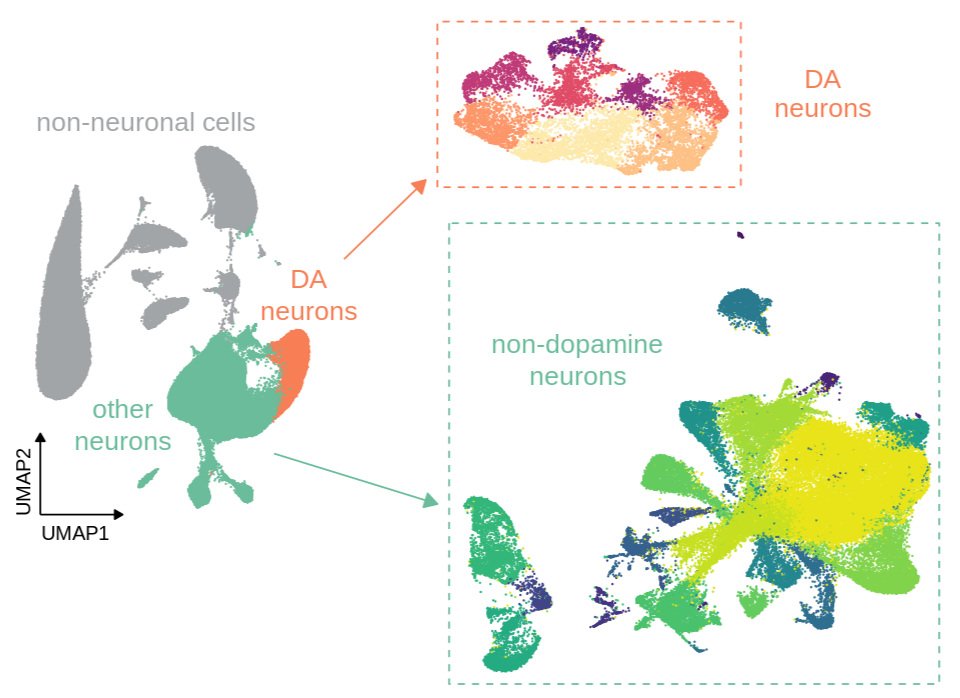
Current projects are investigating the role of chromatin modifications in regulating these patterns of transcriptional change and response to future stress. Using a “Mod-Spec” approach, we have identified a number of long-lasting chromatin modification changes in the VTA, including for marks that have a gene “priming” role in immunology and stem cell biology. Current projects are determining whether these marks have a similar gene priming role in stress sensitivity and psychiatric disease. We are applying a combination of powerful proteomic, snRNA-seq, ATAC-seq, microC and bioinformatic approaches to understand how early life stress alters brain development through transcriptional and epigenetic processes.
Causal testing of predicted molecular mechanisms in vivo
We also use genome-wide sequencing approaches as discovery tools to identify novel molecular mediators of stress susceptibility. Using the mouse two-hit stress paradigm we can then test causality of predicted molecular mediators.
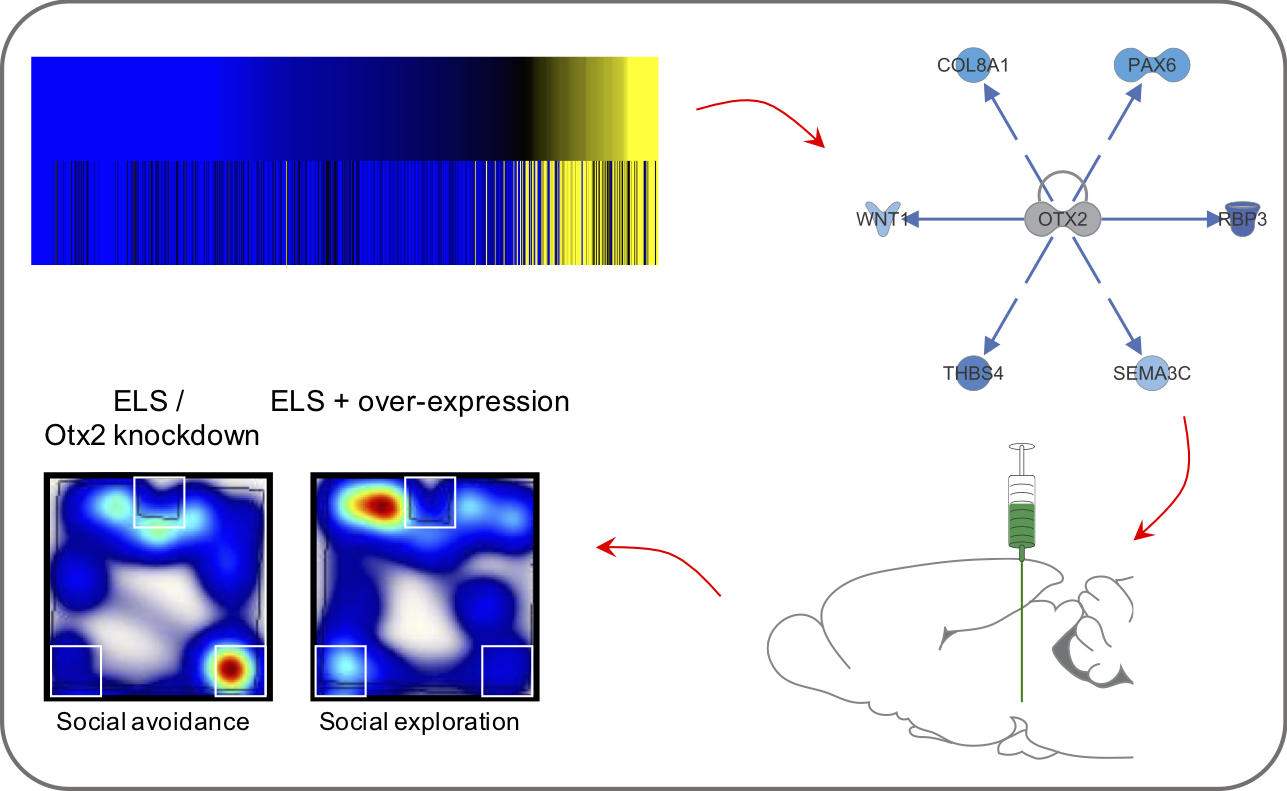
Within the male ventral tegmental area (VTA), bioinformatic analysis predicted the transcription factor OTX2 as an upstream regulator of enduring transcriptional consequences of early life stress. Using bidirectional viral-mediated gene transfer at different developmental stages, we then identified a causal and temporal relationship between VTA Otx2 levels and stress susceptibility in mice, showing that there is a postnatal sensitive window for down-regulation of Otx2 to cause enduring stress sensitivity.
How does early life stress prime sensitivity to future stress at the cellular level?
In parallel, we are asking how early life stress primes sensitivity to future stress or drugs of abuse at the cellular level. Experience has been shown to facilitate formation or strengthening of cellular microcircuits, or ensembles, which are reactivated during recall of the experience. Our work is investigating how early life stress may prime cellular assemblies in mesocorticolimbic regions to be reactivated by adult stress or other salient stimuli, using transgenic mice that allow us to indelibly tag, track, manipulate, and isolate early life stress-responsive cells.
Peña Lab alumna Anne Elizabeth Sidamon-Eristoff conducted research on how the experience of early life trauma sensitizes Central American and Mexican children to stressful immigration experiences such as detention in ICE and CBP facilities at the southern United States border. Listen to her discuss her powerful and award winning research for Princeton Research Days here or at PRADA Grand Rounds here.
We are also collaborating with the Mallarino Lab to understand the impact of early environment on parental behavior in African Striped Mice. This non-traditional model species provides a unique opportunity to examine the neural and epigenomic basis of paternal and bi-parental behaviors that normal lab mice do not show, in a fully-sequenced organism.